General Discussion and Concluding Remarks
Table of ContentsWhen trying to explain the distribution pattern of given phytoplankton species from their ecophysiological characteristics, one should have knowledge about species-specific responses to variations and fluctuations in the growth-determining resources. As inorganic phosphorus (Pi) is often found to be the limiting nutrient for phytoplankton growth during the summer period in both oligo- and eutrophic lakes (e. g. Kalff & Knoechel 1978, Forsberg & Ryding 1980), this nutrient was chosen for the experiments. This thesis is focused on kinetic characteristics of three planktonic desmid taxa (with a roughly comparable cell size) in respect to phosphorus (P) uptake and resulting growth under various P-limited conditions. The three taxa reach maximum cell densities at the end of the summer period, but are found in lakes of different trophic state: Cosmarium abbreviatum var. planctonicum is characteristic of meso-oligotrophic lakes, Staurastrum pingue peaks in meso-eutrophic lakes and S. chaetoceras is almost confined to eutrophic sites (Coesel 1991, 1997). Unfortunately, mainly hypothetical natural conditions can be discussed, because Pi concentrations in the water phase are generally too low to be measured.
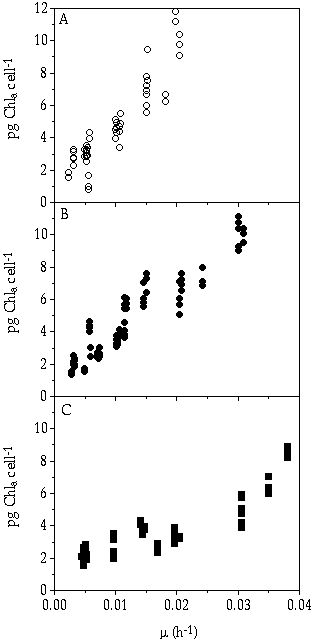
During the summer period, the epilimnion of deep, oligo-mesotrophic lakes can be considered to be almost continuously Pi limited (Forsberg & Heyman 1984), because hardly any recirculation of P across the metalimnion occurs (Caraco et al. 1992). In a continuously Pi limited habitat, those algal species with a relatively high affinity for this nutrient (high maximum growth rate (Ámax)/affinity constant for growth (Ks)), can realize relatively high growth rates and may therefore dominate over other phytoplankton species. Such species may be named affinity specialists, sensu Crowley (1975). Comparing C. abbreviatum with S. chaetoceras and S. pingue, C. abbreviatum can grow faster under stringent Pi-limited conditions ([Pi] < 0.02 ÁM) and consequently wins competition from the other two species under these conditions (chapters 2 & 3). So, C. abbreviatum may be considered an affinity strategist relative to S. chaetoceras and S. pingue. Although the distribution pattern of S. chaetoceras is somewhat different from that of S. pingue, P -uptake kinetics were found to be closely similar (chapter 3). This might be a result of the fact that both clones used in the experiments were isolated from eutrophic sites. However, differences in other characteristics were found such as a higher chlorophyll-a content in S. pingue as compared to S. chaetoceras, (Figure 8-1) supporting the assumption that they are indeed different taxa.

Figure 8-1. Cellular chlorophyll-a contents at different steady-state growth rates. A) C. abbreviatum, B) S. pingue, C) S. chaetoceras.
In shallow, eutrophic lakes large P pulses are known to occur on a regular basis as a result of resuspension of bottom particles by wind action (Boström et al. 1982). This pulsed input of nutrients will be rapidly taken up by the phytoplankton and the enhancement of P supply to a single algal cell will therefore be rather short. A heterogeneous medium favours algal species that are able to take up P fast (Turpin & Harrison 1979, Sakshaug & Olsen 1986). When comparing the three desmid species under discussion, S. chaetoceras and S. pingue have a much higher initial maximum uptake rate (Vi,max) than C. abbreviatum (chapter 3). Therefore, S. chaetoceras (and likely, S. pingue as well) is a better competitor under a broad range of pulsed P-limited conditions (chapters 3 & 5). Compared to C. abbreviatum, S. chaetoceras and S. pingue are so-called velocity specialists (Crowley 1975), having both a high Vi,max and high Ámax (chapter 3 & 7, Sommer 1989). Also, under less stringent, continuous P limited conditions ([Pi] > 0.02 ÁM) S. chaetoceras and S. pingue win competition from C. abbreviatum. This is in accordance to what can be predicted from their species-specific Monod curves, relating growth rate to Pi concentration (crossing Monod curves, chapter 3). In both species, Vi,max hardly varied with growth rate (Á), indicating minimal feedback of internal P quota (Qp) on uptake (Figure 8-2). This was most obvious in C. abbreviatum. In S. chaetoceras some feedback seems to be present. A comparable conclusion can be drawn from uptake rates over a prolonged period (80 h for C. abbreviatum and 50 h for S. chaetoceras) during a saturating P addition (Figure 8-3, results calculated from chapter 4): feedback of Qp on P uptake rate was much less in C. abbreviatum than in S. chaetoceras.
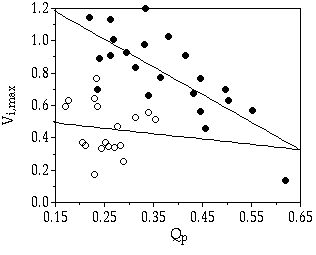
Figure 8-2. Maximum
initial uptake rates (Vi,max,
in Ámol P mg protein-1
h-1)
in relation to internal P quota (Qp,
in Ámol P mg protein-1).
Relation indicated by linear regression. (![]() ) S. chaetoceras (R2
= 0.57), (
) S. chaetoceras (R2
= 0.57), (![]() ) C.
abbreviatum (R2
= 0.01).
) C.
abbreviatum (R2
= 0.01).
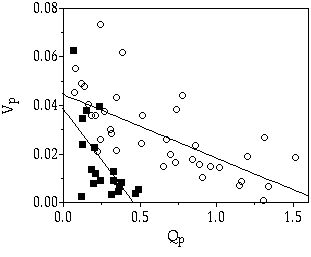
Figure 8-3. P uptake
rates (Vp,
in pmol P cell-1
h-1)
in relation to internal P quota (Qp,
in pmol P cell-1).
Relation indicated by linear regression. (![]() ) S. chaetoceras (R2
= 0.41), (
) S. chaetoceras (R2
= 0.41), (![]() ) C.
abbreviatum (R2
= 0.44).
) C.
abbreviatum (R2
= 0.44).
Perturbations of P concentration can be caused by zooplankton or fish excretion (e.g. Sommer 1989) or inflowing stream water (Powell et al. 1975). Those are considered important P sources for phytoplankton growth in oligo-mesotrophic lakes (Lehman 1980, Gutelmakher & Makartseva 1990, Vanni & Layne 1997). As P pulses are probably less fast consumed in oligotrophic than in eutrophic systems, because of a much lower concentration of primary producers, pulses might be effective over a prolonged period. In addition to that, with the onset of autumn, large mixing events in deep oligotrophic lakes can take place by internal seiches (Kersting 1981) and storms (Marra et al. 1990, Aoki et al. 1996). Such large, incidental P upwelling from the metalimnion can result in an increased P concentration in the epilimnion lasting for days (Imboden et al. 1983). With increasing pulse size and pulse duration, the species' ability to accumulate large amounts of P is probably strategically more important than its capacity for rapid uptake (Reinertsen et al. 1986). This shift in kinetic characteristics determining the outcome of competition is illustrated in chapter 5. The rate of replacement of C. abbreviatum by S. chaetoceras during the competition experiments varied with pulse size and frequency. S. chaetoceras was the superior competitor under intermediately-sized pulses. C. abbreviatum took up P faster than S. chaetoceras when uptake was measured over a longer period (20 to 30 min., Vlt,max) and, as compared to the latter species, it could store 3 times as much P inside the cell (Q'max) after a saturating P addition (chapter 4). After a single, saturating perturbation, growth in C. abbreviatum started later, and the realized initial growth rate was lower than in S. chaetoceras. On the other hand, in C. abbreviatum growth on internal stock can proceed over a much longer period than in S. chaetoceras. When intervals between saturating pulses exceed the period that S. chaetoceras can grow on its internal stock, competition between the two desmids will probably turn out in favour of C. abbreviatum (chapter 5). This is illustrated by a model where 4 hypothetical species compete for a large P pulse, with different time intervals between pulses (12, 25, 100 or 150 days). Uptake and growth on the large pulse follow the variable internal stores model described in chapter 4: uptake rates follow Michaelis-Menten kinetics, growth starts when Q'max is reached or when the pulse is exhausted and follows the Droop model. The species' characteristics used in the model (Table 8-I) only vary in Q'max. The model was tested for a death rate of 1 %, assuming that algal cells disappear from the photic zone with a rate corresponding to a certain percentage of the biomass present.
Table 8-I. Characteristics used in the variable internal stores model estimating cell production and internal P quota of 4 hypothetical species originating from a P-limited environment competing under different pulsed P conditions.
| Species 1 | Species 2 | Species 3 | Species 4 | |
| Vmax (pmol cell-1 h-1) | 0.035 | 0.035 | 0.035 | 0.035 |
| Km (ÁM) | 2.5 | 2.5 | 2.5 | 2.5 |
| Q0 (pmol cell-1) | 0.027 | 0.027 | 0.027 | 0.027 |
| Q'max (pmol cell-1) | 0.1 | 0.2 | 0.4 | 0.8 |
| Ámax (h-1) | 0.040 | 0.040 | 0.040 | 0.040 |
With increasing pulse interval, the species with the highest Q'max wins competition (Figure 8-4). Therefore, Q'max (and with that, the lag period in growth after a pulse, t) is an important competitive characteristic when large, saturating pulses occur and competition largely depends on growth from internal stores.
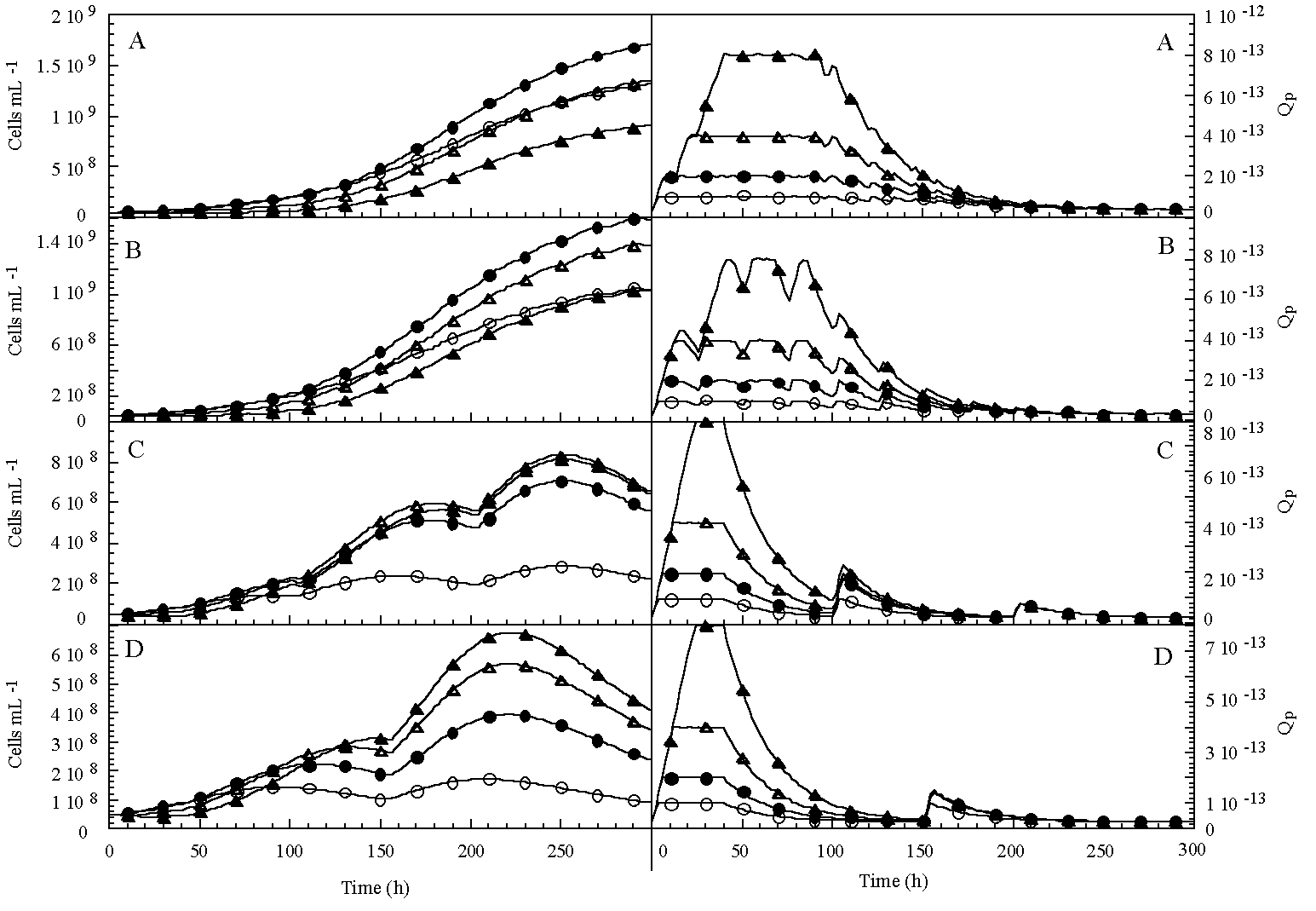
FIGURE 8-4 Evolution
in cell density (Cells mL-1)
and internal P quota (Q)
in 4 hypothetical species at 4 different P pulse regimes: A) 25 ÁM P every 12
h, B) 50 ÁM P every 25 h, C) 100 ÁM P every 100 h and D) 100 ÁM P every 150
h. (![]() ) species 1,
(
) species 1,
(![]() ) species 2, (
) species 2, (![]() )
species 3 and (
)
species 3 and (![]() ) species 4.
) species 4.
The experiments in chapter 4 & 5 emphasize the importance of characteristics as storage (R) and t, rarely studied in growth kinetic research. Usually, storage is determined by comparing cellular P quota from non P-limited conditions (Qmax) with theoretical or measured minimum P quota (Q0) (Tilman & Kilham 1976, Brown & Button 1979). However, in this thesis storage is actually measured from P uptake after a saturating pulse. Only a few comparable data are available from literature (Braddock & Brown 1994, Ducobu 1998). These indicate that higher storage capacities than those presented in other studies might be valid (consult review given in Schreurs 1992). Therefore, storage capacities might have been underestimated in previous studies. A lag phase in growth is thought to be an important phenomenon after a large P addition (Nyholm 1978, Burmaster 1979, Grover 1991b) but only few quantitative data are known (Azad & Borchardt 1970). Chapters 4 & 5 give some insight in how P limitation and pulse dose influence t. The results indicate that after starvation a longer t follows than after continuous P-limited conditions (chapter 5). With increasing pulse size, t increases until a maximum is reached (tmax, chapter 4). This tmax is species specific and depends on the physiological state of the cells as determined by preceding culture conditions. It was found that the length of t equals the time of P uptake: cells took up P until either the pulse was exhausted or Q'max was attained (chapter 4).
Besides inorganic P, organic P compounds (Po) can be important for the stimulation of phytoplankton growth. When Pi in the water column is low, the use of Po will be stimulated. Because most algal species cannot take up Po directly, phosphatase enzymes are synthesized. They hydrolyse the ester bond in Po, releasing Pi for possible uptake by the algal cell. Pi limitation in algal cells leads to an increased phosphatase activity (Healey 1973). Under alkaline conditions, mainly alkaline phosphatase (AP) enzymes are involved. It was found that C. abbreviatum had both a higher maximum APactivity (APAmax) and a higher affinity for the hydrolysation of the organic substrate, methylfluoresceinphosphate than S. chaetoceras (chapter 6). This difference was present under both continuously and pulsed Pi-limited conditions. In the latter situation, APA was measured in cells collected just before as well as just after the pulse. It was shown that enhanced internal P quota in S. chaetoceras caused a decrease in APA, whereas in C. abbreviatum no difference between APA before and after the pulse could be detected. Another difference between the species was found when the dissolved APA fraction was compared in cultures before the moment of pulsing. Cells of S. chaetoceras secreted more enzymes into the medium (resulting in about 80% dissolved APA as compared to total APA) than C. abbreviatum that released only 25% of total APA. The results indicate that C. abbreviatum produced more APA in reaction to a Pi limitation and released less of this production into the surrounding medium than S. chaetoceras (chapter 6). Furthermore, the APA produced in C. abbreviatum was not repressed by temporarily enhanced Pi concentrations. This in contrast to the repressed APA in S. chaetoceras after a Pi pulse. These enzyme activities are only indicative of a possible use of organic P compounds by algal species for growth. Therefore competition experiments were performed between C. abbreviatum and S. chaetoceras under two different organic P limited conditions (chapter 6). Sodiumglycerophosphate was used as a substrate. Under a continuously Po limited condition, C. abbreviatum won competition as could be expected from the species-specific APA measurements: the affinity for hydrolysation of Po is much higher in this species. Under pulsed Po limited conditions, on the other hand, S. chaetoceras won competition. The latter finding was in contrast with the outcome of the APA experiments: C. abbreviatum had a much higher APAmax. When Pi uptake and Po hydrolysation rates, calculated from data obtained in continuous and pulsed Pi-limited cultures, are compared, Pi uptake rates in C. abbreviatum are much lower than its Po hydrolysation rates (chapter 6). This suggests that the outcome of competition for this organic P source can be explained from the uptake kinetics of Pi. From these results it can be predicted that C. abbreviatum would win competition under continuously Po limited conditions because of its higher affinity for Pi and S. chaetoceras would win under the pulsed Po limitation given because of its higher Vi,max.
Cosmarium abbreviatum is characterized by a copious mucilage sheath around its cell, whereas Staurastrum pingue and S. chaetoceras are do not have such a sheath (Coesel 1994). This difference results in a higher biomass weight per unit of P in C. abbreviatum compared to the two Staurastrum species (chapter 3). Even the slightly higher biomass yield of S. pingue as compared to S. chaetoceras might be related to this feature since cells of S. pingue showed some mucilage layer whereas cells of S. chaetoceras completely lacked one. The size of the mucilage sheath increased when C. abbreviatum was grown under pulsed Pi-limited conditions as compared to a continuous Pi limitation. Apart from that, cell size itself increased in both C. abbreviatum and S. chaetoceras under pulsed conditions. The increase in cell size might be an indication of enlarged storage capacity as the limiting nutrient appeared in pulses, whereas the increased mucous layer might be indicative of increased sorption or storage. A question posed in this thesis was if this mucilage envelope could be related to the capture of P. There are some possibilities as to how a mucous layer can be used in trapping P from the water column. One possibility is that the mucous layer binds P non-actively (sorption, Whitton 1967). Besides, P may be stored in the mucous layer (Rorem 1955, Whitton 1967, Decho 1990). Another hypothesis is that bacteria, living in the mucous, provide the algal cell with P released by their metabolism. Enhanced sorption of P was detected in C. abbreviatum (chapter 6). This enhancement was caused by the presence of a mucous envelope, but was not proportional to the increased surface caused by that mucous layer. Sorption of P was found to be < 0.5 % of active Pi uptake in both desmid species. Apart from this finding, no clear indication could be obtained that P is stored in the mucous envelope during a P pulse (chapter 6). Also, after repeated close inspections under the fluorescence microscope it was concluded that bacteria were not present in the mucous envelope. When comparing APA and Vi,max in C. abbreviatum it was found that the maximum hydrolysation rate of organic P substances is much higher than the Vi,max of resulting Pi. This could indicate that part of the phosphatase enzymes are located in the mucous sheath. The high cell-bound APA, as found in C. abbreviatum, may be of particular benefit in an oligotrophic habitat where incidental Po pulses (from e.g. vertebrate excrements) are not readily consumed by a dense bacteria population.
The observed species-specific differences in P uptake and growth kinetics fit very well with the origin of the algal strains used. The kinetics might reflect the 'Ghost of competition past' (Connell 1980, Sommer 1989) when considering P limitation an important factor determining phytoplankton species composition in a given habitat.
In conclusion, C. abbreviatum is characterized by kinetic features that are of particular benefit in an oligotrophic environment, i.e.;
1) a high affinity for both P uptake and growth, enabling a relatively high growth rate under stringent P limitation,
2) a high maximum long-term P uptake rate and, related to that, a high P storage capacity, resulting in a large growth potential after incidental big P pulses and,
3) a high maximum cell-bound alkaline phosphatase activity and a high affinity for the substrate enabling maximum benefit from permanently low concentrations of organic P compounds as well as incidental, locally high pulses.
In contrast to that, S. chaetoceras is marked by kinetic characteristics that favour its occurrence in eutrophic environments, i.e.;
1) a high initial maximum P uptake rate and a high maximum growth rate, rendering a high competitive ability relative to other fast-growing phytoplankton species and
2) a relatively short lag period in growth after saturating P pulses and a relatively high subsequent growth rate after such a pulse, being a good adaptation to frequent fluctuations in dissolved nutrient contents.
Finally, when light is considered to be a potentially limiting resource, it has to be concluded that also in respect to this factor S. chaetoceras is better adapted to a eutrophic environment than C. abbreviatum. As a result of its higher photosynthetic capacity it can profit better by temporary improvements of the light climate as experienced when passing the steep light gradient in the epilimnion of eutrophic lakes (chapter 7).
Top of document Table of Contents