Alkaline phosphatase activity in two planktonic desmid species and the possible role of an extracellular envelope
Elly Spijkerman & Peter F.M. Coesel
Abstract
Introduction
Materials and methods
Results
Discussion
Published in Freshwater Biology
Cosmarium abbreviatum var. planctonicum, a desmid from oligo-mesotrophic lakes, had a higher maximum alkaline phosphatase activity (APA) and affinity constant under both continuous and pulsed inorganic phosphorus (Pi) limitation than Staurastrum chaetoceras, a desmid predominantly encountered in eutrophic lakes. APA of both species increased when measured in starved cells subjected to pulsed Pi condition when compared to continuous Pi limitation. The portion of extracellular relative to cellular APA was higher in S. chaetoceras than in C. abbreviatum, indicating that S. chaetoceras secreted the enzymes more readily into its environment.
The difference in APA could explain the dominance of C. abbreviatum during competition between these two species under conditions of continuous organic P (Po) limitation, but not the outcome under a pulsed Po shortage. The dominance of S. chaetoceras in the latter experiment can, however, be explained by species-specific Pi-uptake characteristics. After a saturating pulse of Pi no increase in Pi in the extracellular mucous layer of C. abbreviatum was found and it was therefore concluded that the mucilage sheath does not store P. However, the sheath could have a main function as accumulation site of cellular APA, providing the cell with Pi.
When, in the water column, external inorganic phosphorus (Pi) concentrations are very low during summer algal blooms, the degree to which phytoplankton may benefit from organic phosphorus (Po) compounds may be decisive to competition. The importance of dissolved organic phosphorus compounds (DOP) as a P source for phytoplankton growth was raised by Currie & Kalff (1984). However, most algal species can use Po for growth only if hydrolysed by phosphatase enzymes (Cembella et al. 1984). Phosphatase enzymes are mainly located at the periphery of algal and bacterial cells (Kuenzler & Perras 1965, Møller et al. 1975) but also occur free dissolved in the water column (Wetzel 1981). In alkaline lakes, in particular, alkaline phosphatases (AP) can be important. Aaronson & Patni (1976) have already calculated that dissolved and cell-bound phosphatases can provide the necessary Pi for growth of P-depleted algae if organic phosphates are present. Francko & Heath (1979) demonstrated that significant quantities of phosphomonoesters can be detected in lake water. In many lakes the concentration of DOP has been found to be up to double that of soluble reactive phosphorus (SRP) (Wetzel 1983, Hantke et al. 1996b). Pearl & Lean (1976) showed that phytoplankton utilized DOP that had been excreted by bacteria, and Hantke et al. (1996b) calculated that probably 60% of DOP can be hydrolysed by AP and used for algal growth, indicating the importance of Po for algal growth as compared to Pi. Indeed, the results of Hernandez et al. (1996) suggest that Po contributes significantly to the total phosphorus demand of the algae in a mesotrophic lake during summer, as Chróst (1988) concluded for a eutrophic community. Hernandez et al. (1996) showed that, in that same lake, Po was quantitatively more important to the algal than to the bacterial fraction and the same was suggested by high alkaline phosphatase activity (APA) in the algal size fraction in a small meso-eutrophic lake (Cotner & Wetzel 1992).
In both oligotrophic and eutrophic waters, Pi often becomes the limiting nutrient during summer algal bloom and alkaline phosphatase activity will be important to phytoplankton growth. To get more insight into species-specific abilities to grow on organic P sources we compared the role of APA in the desmid species Cosmarium abbreviatum var. planctonicum W. & G.S. West and Staurastrum chaetoceras (Schr.) G.M. Smith, typical of oligo-mesotrophic and eutrophic lakes, respectively. Competition experiments were performed with these species under two different types of organic P limitation. Phosphorus uptake and growth kinetics under Pi-limited conditions were studied in chapter 3. It was concluded that C. abbreviatum had a higher affinity for Pi at low dilution rates (D < 0.012 h-1), whereas S. chaetoceras had an overall higher maximum initial uptake rate. In agreement with these results we found in competition experiments that C. abbreviatum became the dominant species under continuously stringent Pi-limited conditions ([Pi] < 0.02 µM P),whereas S. chaetoceras dominated under discontinuous (pulsed) Pi limitations (chapter 3). Both species are comparable in cell volume, but C. abbreviatum always has a thick mucilage envelope around its cell whereas this structure is absent in S. chaetoceras. The size of the mucous sheath of C. abbreviatum appeared to be modified by culturing conditions; it decreased under severe light limitation (Coesel & Wardenaar 1994) and it increased under pulsed Pi-limited conditions (chapter 5). Coesel (1994) found that desmid species characterized by an extracellular mucilage envelope all originated from oligotrophic habitats. On the other hand, desmids from eutrophic sites never had such a structure. The function of an envelope in algae is not clear yet. Usually it is associated with increased floating ability (Boney 1981, Walsby & Reynolds 1980) or thought to protect the alga against grazing (Porter 1973, Revelante & Gilmartin 1991). For desmids the function of the envelope may be related to the acquiring of scarce nutrients. We therefore studied some possible functions of the mucilage envelope in respect to P uptake kinetics.
Algal isolates— The experiments were performed with Staurastrum chaetoceras, clone AO 36, isolated from the alkaline eutrophic Lake IJmeer and Cosmarium abbreviatum var. planctonicum, clone AO 116, isolated from the alkaline, oligo- mesotrophic Lake Maarsseveen (I). For characteristics of these lakes, see Berger & Sweers (1988) and Swain et al. (1987), respectively. An overview of the main characteristics of both lakes is given in Table 7-I (chapter 7) of this thesis. Both clones are maintained in the desmid collection of the Department of Aquatic Ecology, University of Amsterdam.
Culturing conditions of uni-algal cultures— The species were grown at 20 ± 1 °C in 1 L continuous-flow culture vessels. The inflow medium for continuously Pi-limited culture contained 5 µM Pi. For details of the continuous flow device, see Coesel & Wardenaar (1994) and Figure 1-1 (chapter 1) of this thesis, and for the composition of the medium, see chapter 2. The chemostat cultures were brought into a steady-state (D = 0.005 h-1): algal biomass varying less than 5% over at least two renewals of the culture vessel volume. Besides this, pulsed Pi-limited cultures were run at 0.007 h-1. The inflow medium of these cultures contained no Pi (chapter 3). Pi was added directly in the culture vessel from a sterilized stock solution. A pulse of 10 µM Pi was added once every two weeks, resulting in a total weekly P supply of 5 µM Pi which is comparable to that in continuously Pi-limited cultures at the same dilution rate. Circular fluorescent tubes continuously provided an average photosynthetically active radiation (PAR) in the culture vessel of 140-200 µmol m-2 s-1, which proved to be saturating for growth. Cultures were not axenic but bacterial biomass (estimated by acridine staining and counting under a fluorescence microscope) was negligible (<1% of algal biomass). Algal cells were counted and cell volumes estimated with a Coulter Multisizer.
Alkaline phosphatase activity and analytical methods— The alkaline phosphatase activity (APA) was determined in cells from both continuously and pulsed Pi-limited cultures. Cells from continuously Pi-limited conditions were harvested in steady state. From pulsed Pi-limited cultures samples were taken just before the pulse was given (P-starved cells) and after that all external Pi was taken up by the cells and cellular P quota (Qp) was at the maximum to be attained (P-saturated cells). Samples were centrifuged (1500 g, 10 min.) at room temperature after which the pellet was used for determining cellular AP activity and the supernatant for extracellular activity. After washing, cells were resuspended in P-free Woods Hole medium without Fe-EDTA (see Jansson et al. 1988). The medium was brought to pH 8.5 to optimize enzyme activity. APA was measured at 20 ± 2 °C by adding 3-0-methylfluoresceinphosphate (MFP) to the cells and measuring the release of methylfluorescein (MF) over time on a recorder connected to a Perklin Elmer fluorimeter (Perry 1972). The initial concentrations of MFP ranged from 0.05 to 4.5 µM. In case of the continuously Pi-limited cultures samples from three steady states were used. The determination (in duplicate) of the pulse Pi-limited cultures was repeated 10 weeks later in samples from the continuous flow culture in a comparable condition. The extracellular medium in samples from starved cultures was divided into three fractions. Part of the supernatant was filtered through a 1.0 µm filter and part of the filtrate was again filtered through a 0.2 µm filter. APA in all three fractions was measured at pH 8.5 and 20 °C, using 2.25 µM MFP. Cellular P concentrations were determined in the pellet after centrifugation (1500 g, 10 min); total cellular P was measured after digestion with K2S2O8 and H2SO4 for 1h at 100 °C. The external soluble reactive phosphorus (SRP) was assessed from the supernatant after centrifugation (1500 g, 10 min). Both fractions were analysed according to Murphy & Riley (1962). By dividing total cellular P concentration by cell density, cellular P quota (Qp) were obtained.
Competition experiments— Cells from pulsed Pi-limited monocultures of S. chaetoceras and C. abbreviatum were used to start two competition experiments. One culture was continuously supplied with 5 µM sodiumglycerophosphate (Po), whereas the other received a weekly pulse of 5 µM Po. The mono-ester sodiumglycerophosphate was chosen because preliminary results had shown that both desmids could grow on this P source, obtaining growth rates comparable to those based on Pi (results not shown). It is known that many algal species can use this mono-ester as a P source (Walther & Fries 1976, Whitton et al. 1991). Both competition cultures were run at D = 0.007 h-1 and received the same total weekly dose of P. The stock solution of Po was sterilized by filtration (0.2 µm, nucleopore filter) before use. Other nutrients were as in the Pi-limited cultures. To resemble natural conditions more closely, illumination in the competition experiment was supplied under a 16:8 h LD regime. Po pulses were always given in the light period. Cell numbers were counted one to three times a week using a 1 mL capacity Sedgewick-Rafter cell.
P-uptake experiments with and without the extracellular mucilage envelope— Uptake experiments were performed with culture material from continuously Pi-limited cultures. Culture material was washed once by centrifugation and separated into two parts. One part was resuspended in distilled water and sonicated (5 min for C. abbreviatum and 1 min for S. chaetoceras) followed by centrifugation (1500 g, 5 min) following Surek & Sengbusch (1981). After this treatment, both C. abbreviatum and S. chaetoceras cells appeared undamaged. The pellet, whether containing C. abbreviatum cells stripped of mucous or 'undamaged' S. chaetoceras cells, was resuspended in P-free culture medium. The untreated part of the sample was diluted two-fold with P-free culture medium. All suspensions, containing c. 107 cells L-1, were pulsed with different Pi concentrations comprising 32P as described in chapter 2. Initial Pi concentrations ranged from 0.5 to 30 µM. Cellular 32P contents were determined at t = 0, 30, 60 and 300 s, and initial uptake rates (Vi) for every initial Pi concentration were calculated from linear regression, following Riegman & Mur (1984b). By curve fitting to the Michaelis-Menten equation
Vi = Vi,max * (Pi / (Pi + Km))
maximum uptake rate (Vi,max) and half saturation constant for uptake (Km) were computed.
P storage after a big pulse— Samples from pulsed Pi-limited cultures were taken just before addition of the pulse (starved cells) and subjected to a saturating Pi pulse (see chapter 4, for more detail). At different times after the pulse (1, 2 or 3 d), mucous was removed from part of the cells as described in the Pi-uptake experiments. All fractions, after destruction, were measured for P content.
Passive Pi binding experiments— Non-enzymatic Pi uptake was studied with cells from continuously Pi-limited cultures. One part of the sample was sonicated to remove the mucous envelope (as described in the Pi-uptake experiments). The other, untreated part was diluted two-fold with P-free culture medium. To stop biological activity, samples were incubated for half an hour on ice and in darkness and kept under those circumstances during the course of the experiment. Samples were pulsed with 10 µM Pi labelled with 32P. Cellular 32P contents were determined at t = 0, 1, 2 and 3 h.
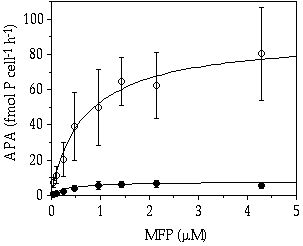
When grown under severe and continuous Pi-limitation, C. abbreviatum had both a higher maximum enzyme activity and a higher affinity for the organic P substrate MFP than S. chaetoceras (Figure 6-1).

Figure 6-1. Alkaline phosphatase activity (APA) in Cosmarium
abbreviatum (![]() ) and Staurastrum
chaetoceras (
) and Staurastrum
chaetoceras (![]() ) from continuously
P-limited cultures, in relation to the concentration of added 3-0-methylfluoresceinphosphate
(MFP).
) from continuously
P-limited cultures, in relation to the concentration of added 3-0-methylfluoresceinphosphate
(MFP).
When the cells were grown under a pulsed Pi limitation in both species an increased APA was observed, probably induced by the long period of starvation before the pulse (Figure 6-2). The difference in APA between the species under continuous Pi limitation persisted under Pi-starved conditions; C. abbreviatum having both a higher maximum APA as a higher affinity for MFP than S. chaetoceras. After a pulse, however, when the cells were saturated with Pi, APA in S. chaetoceras was decreased whereas the activity in C. abbreviatum remained constant (Figure 6-2).
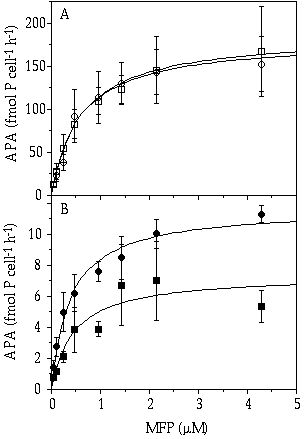
Figure 6-2. Alkaline phosphatase activity (APA) in Cosmarium abbreviatum (A, open symbols) and Staurastrum chaetoceras (B, closed symbols) from continuous flow cultures before (circles, P-starved cells) and after (squares, P-saturated cells) a pulsed addition of phosphate in relation to the concentration of added 3-0-methylfluoresceinphosphate (MFP).
The extracellular AP in these pulsed Pi-limited cultures in starved condition was mainly present in dissolved form (fraction < 0.2 µm, Table 6-I). Activity ascribable to bacterial contamination (0.2 <APA< 1.0 µm) was neglectable (Table 6-I). In S. chaetoceras, the extracellular APA was over 80% of total APA whereas it was only about 25% in C. abbreviatum (Table 6-I). Because this external activity was not bound to the bacterial fraction, it was probably secreted by the algae. We conclude therefore that S. chaetoceras secreted much more of its produced AP into the culture medium than C. abbreviatum.
Table 6-I. Alkaline phosphatase activity (20 °C, pH 8.5, in fmol P cell-1 h-1) in the medium surrounding starved C. abbreviatum and S. chaetoceras cells taken from continuous flow cultures under a pulsed P regime just before the pulse and the percentage (%) of this secreted activity compared to the total activity in the culture. APA was measured in different fractions (by filtration) provided with 2.25 µM MFP as a substrate. Average ± SD, number of replicates between parentheses.
|
fraction |
C. abbreviatum |
% |
S. chaetoceras |
% |
|
extracellular |
50.9 ± 3.4 (4) |
26.3 |
44.2 ± 7.6 (8) |
81.5 |
|
< 1.0 µm |
55.9 ± 7.6 (4) |
28.1 |
44.5 ± 6.3 (8) |
81.6 |
|
< 0.2 µm |
44.1 ± 1.1 (4) |
23.6 |
43.2 ± 5.1 (8) |
81.2 |
When competing for a continuously supplied organic P compound C. abbreviatum displaced S. chaetoceras in a 30 day period (at a rate of 0.023 d-1, Figure 6-3A). When C. abbreviatum and S. chaetoceras competed under pulsed Po-limited conditions, S. chaetoceras was able to maintain its population density in the culture vessel, whereas C. abbreviatum was outcompeted (at a rate of 0.019 d-1, Figure 6-3B). In both competition experiments, bacterial contamination increased with time. At the end of the continuously Po-limited experiment, bacterial biomass was still relatively low, i.e. less than 5% of total biomass. In the pulsed Po-limited culture, however, bacterial growth was more substantial, as was evident from macroscopical flocks appearing after some weeks. Because of inhomogeneous dispersion of these flocks bacterial biomass was hard to quantify, the decreased algal biomass production in this culture after 25 days may indicate that bacterial production was important from this point onwards (Figure 6-3B).
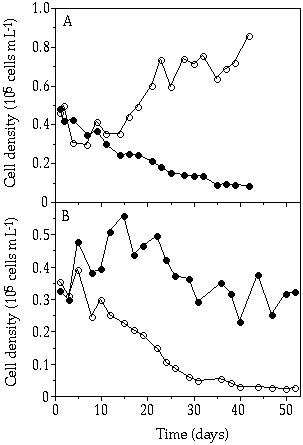
Figure 6-3. Cell densities of Cosmarium abbreviatum
(![]() ) and Staurastrum chaetoceras
(
) and Staurastrum chaetoceras
(![]() ), growing in continous flow-cultures
under a continuously organic P limitation (A) or a pulsed organic P limitation
(B) in relation to time.
), growing in continous flow-cultures
under a continuously organic P limitation (A) or a pulsed organic P limitation
(B) in relation to time.
After sonication of Pi-limited cells, maximum Pi-uptake rates in C. abbreviatum or S. chaetoceras did not change (Table 6-II). Sonication, however, caused a distinct decrease in the affinity for Pi uptake in both species, particularly in S. chaetoceras (Table 6-II).
Table 6-II. Relative increase or decrease in maximum initial uptake rate (Vi,max) and affinity for uptake (Vi,max/Km) in Pi-limited C. abbreviatum and S. chaetoceras after sonication as compared to values in untreated cells. Average percentages ± SD, number of replicates given between parentheses.
|
|
C. abbreviatum |
S. chaetoceras |
|
Vi,max |
101 ± 36 (5) |
101 ± 2 (2) |
|
Vi,max/Km |
68 ± 35 (5) |
40 ± 3 (2) |
When Pi-starved cells of C. abbreviatum and S. chaetoceras were subjected to a saturating pulse, resulting into an enhanced concentration for successive days, part of cellular P could be sonicated off the cells of both species. No indication could be found that C. abbreviatum stored P from the pulse in its mucilage layer (Table 6-III). From the S. chaetoceras cells more P could be sonicated than from cells of C. abbreviatum indicating (again) a higher sensitivity for sonication in S. chaetoceras.
Table 6-III. Percentage of the total cellular P concentration of C. abbreviatum and S. chaetoceras cells after a saturating pulse that is lost by sonication. Average ± SD, number of replicates between parentheses.
|
C. abbreviatum |
S. chaetoceras |
|
9.64 ± 8.64 (4) |
22.83 ± 8.03 (5) |
The passive binding rate of Pi when expressed per cell or cell surface area (microscopically determined by use of appropriate geometric formulae, measurements used from chapter 5) was higher in C. abbreviatum than in S. chaetoceras (Figures 6-4A, C). When sorption was expressed per biovolume, measured under the microscope, the difference between the species was smaller (Figure 6-4B), and disappeared totally when expressed per biovolume, as measured with a Coulter counter (Figure 6-4D). The biovolume measurements on the Coulter are known to include some part of the mucous layer (about 0.5%) as C. abbreviatum cells were bigger when measured on the Coulter compared to measurements done under the microscope and this difference was not found with cells of S. chaetoceras (chapter 5). When sorption was expressed per unit of area or biovolume of mucous layer in C. abbreviatum (microscopic measurements used from chapter 5), sorption was much lower in C. abbreviatum compared to S. chaetoceras indicating that the mucous layer does not enlarge cellular sorption capacity proportionally (Figures 6-4B, C). When the passive binding rate of C. abbreviatum cells separated from their mucous layer was compared with untreated S. chaetoceras cells (Figure 6-5) no difference could be detected (ANCOVA, F = 4.615, P > 0.01) between these sorption rates.
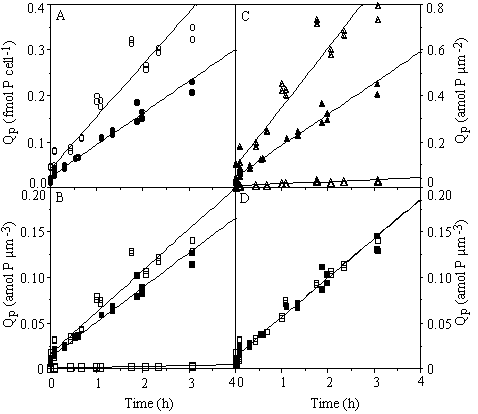
Figure 6-4. Cellular P quota (Qp) in Cosmarium abbreviatum (open symbols) and Staurastrum chaetoceras (closed symbols) originating from steady state Pi-limited continuous cultures (µ = 0.005 h-1), obtained after a pulse of 10 µM P in relation to time. Cells were kept on ice and in the dark. Qp expressed per cell (A), on cell volume as measured under the microscope (B), on cell surface area as measured under the microscope (C), and on cell volume as measured by the Coulter counter (D). The larger open symbols in B and C refer to calculation done including volume (B) and surface area (C) of the mucous layer in C. abbreviatum.
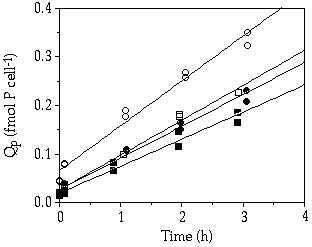
Figure 6-5. Cellular P quota (Qp) in Cosmarium abbreviatum (open symbols) and Staurastrum chaetoceras (closed symbols) originating from steady-state Pi-limited continuous cultures, obtained after a pulse of 10 µM P in relation to time. Cells were put on ice and in the dark and part of the sample was sonicated to remove the extracellular mucilage layer of C. abbreviatum. Qp expressed on cell basis for untreated cells (circles) and sonicated cells (squares).
In our experiments with C. abbreviatum and S. chaetoceras growing on the organic P substrate sodiumglycerophosphate, hydrolysation of this organic compound is assumed to be a first step. Under both continuously Pi-limited and periodically starved conditions maximum activity as well as affinity for the hydrolysation of the organic substrate MFP was much higher in C. abbreviatum than in S. chaetoceras. If, in this respect, MFP and sodiumglycerophosphate result into comparable metabolic activities, these characteristics will give C. abbreviatum a competitive advantage over S. chaetoceras under both continuously and pulsed Po-limited conditions. When Po was permanently limiting, C. abbreviatum indeed dominated the mixed culture for 90% after 30 days. Hydrolysation of sodiumglycerophosphate appeared to be such a fast process (completed within 3 minutes, results not shown) that it remains unclear whether the high affinity for hydrolysation of Po or the high affinity for Pi-uptake was determining the competitive success of C. abbreviatum. For in both parameters C. abbreviatum is superior to S. chaetoceras.
Contradictory to our expectations, C. abbreviatum did not become dominant in the pulsed Po competition experiment. In trying to explain the outcome of the latter experiment, we tested some three hypotheses about P uptake characteristics in C. abbreviatum. The first one states that C. abbreviatum can hydrolyse Po much faster than it can subsequently absorb the liberated Pi. This would imply a temporary overproduction of Pi near cells of C. abbreviatum. Indeed, by calculating rates of hydrolysation and uptake, an 'overproduction' of Pi by C. abbreviatum was found (Table 6-IV). On the other hand, cells of S. chaetoceras cannot hydrolyse enough Po to meet their Pi uptake capacity (Table 6-IV).
Table 6-IV. Initial P uptake rate of 5 µM Pi and hydrolysis rate of 5 µM Po (fmol P cell-1 h-1) in C. abbreviatum and S. chaetoceras under either continuously or pulsed (starved) Pi-limited conditions. Average ± SD, number of replicates between parentheses (uptake rates calculated from chapter 3 (cont. Pi-lim.) and chapter 5 (pulsed Pi-lim.)).
|
|
uptake of 5 µM Pi |
hydrolysis of 5 µM Po |
||
|
|
(cont. P-lim.) |
(pulsed P-lim.) |
(cont. P-lim.) |
(pulsed P-lim.) |
|
C. abbreviatum |
40 ± 6 (6) |
89 ± 15 (11) |
80 ± 26 (7) |
152 ± 32 (3) |
|
S. chaetoceras |
54 ± 7 (5) |
104 ± 13 (8) |
6 ± 2 (6) |
11 ± 1 (4) |
The second hypothesis is that transport of Pi is not delayed by the mucous layer of C. abbreviatum and that 'overproduced' Pi can diffuse freely into the medium (no-barrier hypothesis). Directly related to this, a third hypothesis was considered that proposed that Pi is not stored in the mucous layer of C. abbreviatum (no-storage hypothesis). In general, a mucous layer is often assumed to be a diffusion barrier for nutrients (Wetzel 1983, p.358), although there are few experimental data. Chang (1980) removed the mucilage sheath from Oscillatoria rubescens D.C. with an enzyme solution and found that CO2 uptake was enhanced by this removal. Veldhuis et al. (1991) found, by comparing Vi,max and Km of colonies and single cells, that in Phaeocystis pouchetii (Hariot) Lagerh. (syn.: P. globosa Scherffel) the colonial mucous is a diffusion barrier for the uptake of P. In contrast to this, the mucous layer around Phaeocystis pouchetii cells appeared to be no diffusion barrier for 3-0-methyl-fluorescein phosphate as used for APA measurements (Veldhuis & Admiraal 1987). In our experiments, removement of the mucilage envelope of C. abbreviatum did not alter maximum uptake rates. Apparently, uptake of high concentrations of Pi was not hampered by the presence of a mucous layer. Unfortunately, an observed decreased affinity for Pi uptake in sonicated cells of both species suggests that sonication caused some damage to the cell membrane, so that we cannot draw any conclusion on a possible diffusion limitation by the mucilage layer at low Pi concentrations. It is true, in some phytoplankton species, e.g. Synechococcus leopoliensis (Racib.) Kom. (Mierle 1985) and Oscillatoria agardhii Gom. (Riegman & Mur 1984a) where Pi uptake at low concentrations did not follow Michaelis Menten kinetics, diffusion was found to be a limiting factor. However, applying comparable calculation methods (Mierle 1985) we could not find such an indication as to our uptake experiments.
No substantial storage of Pi could be detected in the mucous layer of C. abbreviatum after a saturating pulse. Storage of essential nutrients is often suggested to take place in a mucous envelope or more general in extracellular polymeric substances (Decho 1990). Rorem (1955) showed that the presence of extracellular polysaccharide led to a much greater accumulation of P by several bacteria and that much of the increased P content was associated with the polysaccharide slime. Whitton (1967) found evidence that colonies of Nostoc verrucosum Vauch. can accumulate more Pi from the environment than could be taken up by cells alone. Veldhuis et al. (1991) concluded from indirect evidence that Pi was stored in the colonial matrix of Phaeocystis globosa, although this was subsequently denied by Riegman & Van Boekel (1996). Although we found the P storage capacity to be much larger in C. abbreviatum than in S. chaetoceras whereas cell volumes are comparable (chapter 5) we can not conclude that this difference is caused by P-storage in the extracellular mucous layer of C. abbreviatum.
From our findings we speculate that when organic P is supplied in distinct pulses to a mixed culture of C. abbreviatum and S. chaetoceras, Po will be hydrolysed mainly by C. abbreviatum, but because this species has a relatively low maximum initial Pi uptake rate it can not use all Pi that it has delivered. As Pi is not stored in the mucilage layer, it will escape into the medium and become available to S. chaetoceras. The latter species then profits at the cost of C. abbreviatum, at least in the beginning of the experiment. In both the continuously and the pulsed Po limited competition experiment it is unclear if differences in species-specific APA played any significant role in competition for Po since the outcome can be predicted on the characteristics of Pi-uptake parameters of the individual species alone.
Compared to APA under a continuous Pi-limitation, APA increased in both species when they were starved of Pi. Under these latter conditions there were few bacteria (results not shown). Besides this, the results found for extracellular APA suggest that bacterial activity was very low and therefore did not play a substantial role at the beginning of the competition experiments on Po. Bacteria are important producers of APA (Stewart & Wetzel 1982, Hantke et al. 1996b) and can use Po for growth. Hernandez et al. (1996) found that the bacterial and algal fraction from a mesotrophic lake sample had comparable affinities for hydrolysis of Po, but that the algal fraction had a higher maximum APA than the bacterial fraction. Also, other studies indicate that phytoplankton is an important APA producer in lakes (Heath & Cook 1975, Hantke et al. 1996b). Kobori et al. (1979) found that bacteria in coastal waters produced mainly repressible phosphatases, whereas most bacteria producing phosphatase in oceanic water had constitutive enzymes with high activity. These results might relate to our finding that APA was repressed after a P-pulse in S. chaetoceras but high and constitutive in C. abbreviatum (provided that in C. abbreviatum not all APA is located in its extracellular sheath, see below). Regarding their abiotic niche, S. chaetoceras comes from a eutrophic lake and C. abbreviatum from an oligotrophic environment, comparable to a coastal (eutrophic) versus oceanic (oligotrophic) habitat.
One of the possible explanations for the high APA in C. abbreviatum is that APA is accumulated in its mucous sheath. Doonan & Jensen (1977) thought it unlikely that APA was located in the sheath of the cyanobacterium Plectonema boryanum Gom. Livingstone et al. (1983) in contrast, detected acid phosphatase activity in the sheath of a cyanobacterium species. Considering the 'overproduction' of AP in C. abbreviatum, it could be that AP, after secretion by the cell, is trapped in the mucilage layer where it is still active even while the cell is P-saturated. In a preliminary experiment it was found that after sonication and vortexing cells of S. chaetoceras and C. abbreviatum, more APA was released from cells of C. abbreviatum than from S. chaetoceras (results not shown). Another explanation would be that bacteria housing in the extracellular mucous layer, were responsible for the APA. In DAPI-stained samples of C. abbreviatum from a culture growing under continuous Po limitation, bacteria were seen in the medium but they could not be found in the mucous envelope. We therefore conclude that C. abbreviatum itself was responsible for the high enzyme production.
Comparing APA in C. abbreviatum and S. chaetoceras, it can be concluded that C. abbreviatum produces more (constitutive) AP than S. chaetoceras. Moreover, in C. abbreviatum a larger part of this AP production is kept near the cell than in S. chaetoceras. Translating these activities to the field, it can be hypothesized that the species originating from an oligotrophic habitat (C. abbreviatum) invests more cellular energy into the production of AP to use organic P substances for growth. This may be lucrative since dissolved APA in oligotrophic waters, because of low plankton densities, will be relatively low. On the other hand, S. chaetoceras, a species characteristic of shallow, eutrophic lakes with (very) high plankton densities and consequently relatively high total APA, might better invest resources into a high uptake rate of inorganic P pulses.
Although it is clear that a mucilage envelope affects buoyancy of algal cells (Boney 1981, Walsby & Reynolds 1980) and protects them against predation (Porter 1973, Decho 1990), the observation of Coesel (1994) that, among desmids, a distinct mucous layer is found only in species from oligotrophic lakes, strongly suggests that this layer is related to the capture of scarce nutrients. We have shown that the increase of (inactive) sorption of Pi in C. abbreviatum due to a mucous envelope is negligible in relation to active Pi uptake (< 0.5 %). Neither could we demonstrate any substantial storage of Pi in the envelope. However, that the capsule in C. abbreviatum enhances cellular phosphatase activity is still a serious option.